The Devil’s Work: A Maxwell's Demon Model for Understanding the Work Cost of Isotope Enrichment
B. Cameron Reed, Department of Physics (Emeritus), Alma College, camfissile@icloud.com
1. INTRODUCTION
Potential nuclear proliferation remains a concern for policymakers. Followers of proliferation affairs will know that uranium occurs in two isotopes, 235U and 238U, but that only the former can be used to make a bomb. Because these isotopes occur in nature in very unequal proportions (about 0.72% and 99.28%, respectively), it is necessary, if one desires to make a uranium-based fission bomb, to process a supply of ore to enrich the abundance of 235U to up to about 90%. What can seem very counter-intuitive, however, is to learn that much more work is required to raise the enrichment level from the natural level to, say, 20% than is required to go from 20% to 90%. While this has to be qualified with information about the masses involved, it is fundamentally true and is the reason that non-proliferation experts become concerned when a country’s enrichment capacity begins to exceed the 3-5% level typical of reactor fuel. Unfortunately, a rigorous derivation of the expression for the amount of work involved in enrichment is very complex. [1-3] The result of such analyses is an unusual second-order differential equation, the solution of which, Eqs. (8) and (9) below, is not at all obvious. The purpose of this article is to offer a mini-tutorial on enrichment and a simplified model of its energy cost based on imagining a Maxwell's demon-type creature who can sort isotopes by their weight. This treatment should be accessible to any reader familiar with basic concepts of probabilities, and the result gives numerical predictions which prove to be in remarkably good accord with the rigorous result.
Sections 2 and 3 of this paper cover some preliminaries. Uranium is normally a metal, and in the enrichment industry it must first be rendered into gaseous form for processing; the preferred current process is to employ cascades of centrifuges. To this end, the usual working material is uranium hexafluoride, UF6, colloquially known as “hex”. Hex is a powder at room temperature and can be easily stored and transported, but it sublimes directly to a gas when heated to above about 60 C, making it ideal for use in centrifuges. The fluorine is later removed by chemical means to leave behind uranium metal or perhaps an oxide. Section 2 explores the arithmetic of determining exactly what mass of 235U is contained within a given mass of hex that has been enriched to a specified 235U abundance. This is strictly a separate issue from the energy cost of enrichment, but is included here for sake of completeness.
Any enrichment method will require energy to operate: gases must be pumped into and out of centrifuges, which must be spun. For enrichment engineers, the currency of the realm is known as the “Separative Work Unit.” Abbreviated SWU and pronounced “swoo,” these numbers quantify the energy cost of achieving the desired feed-to-product enrichment. SWUs are strictly a measure of energy, but the custom in the trade is to quote them in units of “kg-SWU,” a measure of the energy required to produce one kilogram of hex whose uranium content has been enriched to a specified percentage of 235U. Enrichment facilities are usually characterized by their annual kg-SWU capacities, although some large commercial firms cite their capacities in tonne-SWU per year, where tonne refers to the metric ton of 1,000 kg.
To compute a SWU value requires five elements of input data. These are the number of kilograms of feed material, the number of kilograms of product material, the enrichment level of the feed material, the desired enrichment level of the product material, and the enrichment level of the leftover waste or “tails” material, which will be less than that of the feed material. Section 3 sets up the key relationship between these parameters. The demon-inspired separation model is set up in section 4, its predictions are compared to those of the rigorous SWU expression, and some numerical examples are given. Section 5 offers some comments on current worldwide enrichment capacity.
2. HEX AND 235U MASSES
Fluorine consists of only one isotope, 19F, that is, it has atomic weight 19 grams per mole. In this paper, I ignore the very slight non-integer values of the atomic weights of the elements and isotopes involved. Hex molecules containing 235U atoms will then have an atomic weight of 235 + 6(19) = 349 gr/mol, while those bearing 238U atoms will weigh in at 352 gr/mol. Now suppose that you have a sample of hex where the fraction of molecules containing 235U atoms is f235. The overall atomic weight of the sample will be Ahex = 349 f235 + 352(1– f235), that is, (352 – 3 f235) gr/mol. From basic chemistry, if the mass of the sample is Mhex grams, then the number of moles of hex must be Mhex/Ahex. The number of moles of 235U atoms in the sample will then be f235 (Mhex / Ahex) and the mass of 235U will be this number times 235 gr/mol:
 For a sample of hex that has been enriched to contain only atoms of 235U, the mass of 235U contained therein can never exceed (235/349) = 0.673 times the mass of the sample. To optimize compactness and efficiency, most commercial power reactors operate with fuel enriched to a concentration of typically 3.5% 235U: f235 = 0.035. This is known in the nuclear trade as Low-Enriched Uranium, or LEU. By International Atomic Energy Agency definition, LEU extends up to 20% 235U; beyond this one has Highly-Enriched Uranium, or HEU. Many research and medical isotope-production reactors operate with fuel enriched to near the LEU/HEU transition value. Beyond a 235U content of 90% one has weapons-grade uranium. Some very compact reactors designed for use in naval vessels operate with very highly enriched uranium, but for countries whose nuclear activities are for the purposes of power production there is usually no need to enrich beyond a few percent.
For a sample of hex that has been enriched to contain only atoms of 235U, the mass of 235U contained therein can never exceed (235/349) = 0.673 times the mass of the sample. To optimize compactness and efficiency, most commercial power reactors operate with fuel enriched to a concentration of typically 3.5% 235U: f235 = 0.035. This is known in the nuclear trade as Low-Enriched Uranium, or LEU. By International Atomic Energy Agency definition, LEU extends up to 20% 235U; beyond this one has Highly-Enriched Uranium, or HEU. Many research and medical isotope-production reactors operate with fuel enriched to near the LEU/HEU transition value. Beyond a 235U content of 90% one has weapons-grade uranium. Some very compact reactors designed for use in naval vessels operate with very highly enriched uranium, but for countries whose nuclear activities are for the purposes of power production there is usually no need to enrich beyond a few percent.
3. FEED, PRODUCT, AND WASTE
In an enrichment process, a sample of hex known as the “Feed” is introduced into the operating machinery. Let the number of molecules or mass of feed material be F, and designate its 235U concentration as fF as defined above. From this, the enrichment process is desired to yield mass P of product enriched to concentration fP. Left behind will be W = F – P molecules or kilograms of waste material whose concentration fW will be less than that of the feed material. The waste material is also known as “depleted” in that its content of 235U has been depleted relative to that of the feed material. In the enrichment community, the term “tails” is synonymous with waste; a typical tails concentration is 0.25%, or fW = 0.0025. Figure 1 illustrates the idea of an enrichment process in schematic form.
 For practical purposes, you will probably want to know what mass of product can be obtained from a given mass of hex feed and set of concentrations. An expression for this can be derived by demanding that both mass and number of moles of material be conserved: F = P + W and F/AF = P/AP + W/AW , where the A’s designate atomic weights. Eliminating W from these two expressions leads to
For practical purposes, you will probably want to know what mass of product can be obtained from a given mass of hex feed and set of concentrations. An expression for this can be derived by demanding that both mass and number of moles of material be conserved: F = P + W and F/AF = P/AP + W/AW , where the A’s designate atomic weights. Eliminating W from these two expressions leads to This can be simplified without any significant loss of accuracy. At most, fP can approach unity, so even if fF is very small, the first bracketed term on the right side can never drop below (349/352) = 0.9915. At the other extreme, this term can never exceed unity, so we are quite safe in dropping it and simplifying to
This can be simplified without any significant loss of accuracy. At most, fP can approach unity, so even if fF is very small, the first bracketed term on the right side can never drop below (349/352) = 0.9915. At the other extreme, this term can never exceed unity, so we are quite safe in dropping it and simplifying to  In Eqs. (1) and (3), masses, P, and F are usually assumed to be in kilograms, but can equally well be in numbers of molecules or just a more generic “units”.
In Eqs. (1) and (3), masses, P, and F are usually assumed to be in kilograms, but can equally well be in numbers of molecules or just a more generic “units”.
It may seem strange that we can specify all three of fF, fP, and fW for a given amount of feed material within the obvious constraint that we must have fP > fF > fW. The price paid for this liberty is that for fixed values of fF and fW, the amount of product obtainable from a given amount of feed drops steadily as fP increases; this will be discussed in the context of Fig. 4 below.
Here is an example of using Eqs. (1) and (3) together. Suppose that a putative bomb-making country has 10,000 kg of hex already enriched to 5% 235U. If this is to be enriched in one step to a product of concentration of 90% along with tails of concentration 0.25%, then Eq. (3) indicates that we can expect to recover (10,000 kg)(0.05 – 0.0025)/(0.9 – 0.0025) = 529 kg of product if there are no losses in the machinery. Equation (1) then indicates that this product would have a 235U content of (529 kg)(235)(0.9)/[352 ¬– 3(0.9)] = 320 kg. This would be enough for about five Hiroshima-type Little Boy bombs.
4. ENTER THE DEMON
This section describes a simplified model for the work involved in enrichment.
To begin, imagine that the feed material of F molecules or kilograms and 235U concentration fF is contained within a chamber. This is sketched in Figure 2. The demon begins by inserting a barrier (vertical line) into the chamber to divide it into waste (left, W) and product (right, P) sides, in such a way that the desired number of product molecules P as dictated by Eq. (3) find themselves in the right side, and hence the desired number of waste molecules W remain in the left side. Initially, the number of 235U molecules in the right side will be fF P, whereas what is desired is fF P molecules of this type. This means that (fP - fF)P molecules containing 235U atoms have to be shifted from the W side to the P side, while the same number of molecules containing 238U atoms will have to be shifted from P to W. The demon has work to do.
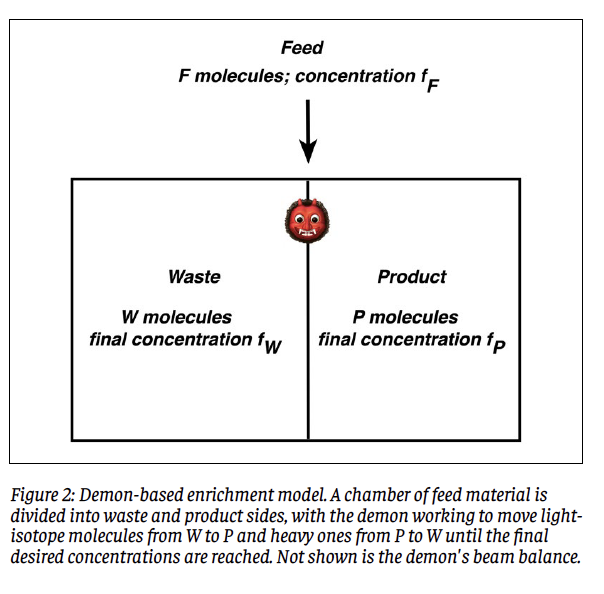
Now, the demon cannot tell lighter from heavier molecules merely by looking at them; he has to weigh them. To this end, we provide him with a beam balance. The fundamental idea is that the demon performs rounds of drawing pairs of molecules from each side and weighing them. Suppose that he starts with the W side. If he draws two light or two heavy molecules, the balance will not be able to discriminate between them and he will have to toss them back and make another draw. At some point he will draw one light and one heavy molecule, and he can set the light one aside for transfer to side P while returning the heavy one to W. He then repeats this procedure on the P side until he isolates a heavy molecule to be shunted to the W side. The two chosen molecules are dumped into their new homes, and the process begun anew for a second round of pair-drawings. Here is the key assumption: That the amount of work performed in any round of drawings is taken to be the inverse of the probability of drawing two dissimilar molecules. The rationale behind this assignment is that a low (high) probability will translate to a larger (smaller) number of pair-drawings, each one of which takes effort on the part of the demon. He continues in this way until the desired final conditions have been achieved, accumulating work along the way. An important point to be accounted for is that the isotopic concentrations will alter ever so slightly upon each round of draws. Since the proportion of sought-after molecules decreases on each side with each round, the work required will increase with each round but the number of remaining required rounds will steadily decrease. The total work required will reflect the competition between these effects. To be sure, one can imagine many hypothetical ways of performing such a separation procedure. A feature of this model is that the density of particles in each side of the chamber remains constant as drawings proceed.
To quantify this, imagine the sequence of draws from the W side of the container. In the first round, the probability of drawing dissimilar molecules will be 2 fF (fF – 1), with the factor of two arising from the possible draw sequences (light, heavy) and (heavy, light). The average number of draws to get dissimilar molecules will be the inverse of this, 1/[2 fF (fF – 1)], which I take to be, upon multiplication by a factor of 2, the work required: Work = 1/[ fF (fF - 1)]. The rationale for the factor of two is that two actions are required in each pair-drawing. I ignore the presumably very slight change in concentrations caused by the selection of the first molecule of a pair.
After the first-round draws have successfully resulted in a swap (the work done on the P side is accounted for below), the demon moves on to the second-round draws for the W side. However, the number of lighter-isotope molecules in W will have been reduced to fF W – 1, while the number of heavies will have increased to (1 – fF)W + 1. The abundance of lights will consequently now be fnew = (fF W – 1) /W = fF – 1/W. The change in concentration between rounds is Δf = –1/W, an expression that will be used shortly. For the second-round draws, the probability of selecting dissimilar molecules will be 2 fnew (fnew – 1), with corresponding work 1/[ fnew (fnew – 1)].
I call the work involved here "Demon Work Units" (DWUs) in analogy to SWUs. Continuing this procedure gives the total work on the W side as  where the index i runs over rounds of draws; fi = fF – (i – 1)/W. To circumvent having to sum over the astronomical number of rounds required to process kilograms of material, it is convenient to turn Eq. (4) into an integral. We can do this as follows. Write the factor of “1” in the numerator of Eq. (4) as – W Δ f from the argument above regarding the change in concentration. This gives
where the index i runs over rounds of draws; fi = fF – (i – 1)/W. To circumvent having to sum over the astronomical number of rounds required to process kilograms of material, it is convenient to turn Eq. (4) into an integral. We can do this as follows. Write the factor of “1” in the numerator of Eq. (4) as – W Δ f from the argument above regarding the change in concentration. This gives For the P side the work emerges similarly, with W replaced by P and limits (fF, fP). The total work is then
For the P side the work emerges similarly, with W replaced by P and limits (fF, fP). The total work is then These integrals are standard. The lower limits give identical results but for the prefactors of W and P, which can be combined as W + P = F. The result is
These integrals are standard. The lower limits give identical results but for the prefactors of W and P, which can be combined as W + P = F. The result is now a rigorous analysis of separative work shows that the correct expression is
now a rigorous analysis of separative work shows that the correct expression is  where V(x) denotes to so-called “ value function”,
where V(x) denotes to so-called “ value function”, Equations (7) - (9) share some similarities, but differ due to the hypothetical procedure imagined here; real enrichment process do not get to pick and choose what molecules they operate on. Perhaps somewhat surprisingly, however, they give remarkably similar numerical results. Figure 3 shows DWU vs. SWU for 13 combinations of (fF, fP), with both evaluated for P = 1 and fw = 0.0025. The correlation is remarkably tight: y = 0.9517x – 1.589 (r 2 = 0.9987). The demon model underestimates SWU by a few percent (for example, 205 vs. 219 at the topmost point), but this can be regarded as acceptable given the pedagogical intent. Similarly good correlation holds for other reasonable tails concentrations.
Equations (7) - (9) share some similarities, but differ due to the hypothetical procedure imagined here; real enrichment process do not get to pick and choose what molecules they operate on. Perhaps somewhat surprisingly, however, they give remarkably similar numerical results. Figure 3 shows DWU vs. SWU for 13 combinations of (fF, fP), with both evaluated for P = 1 and fw = 0.0025. The correlation is remarkably tight: y = 0.9517x – 1.589 (r 2 = 0.9987). The demon model underestimates SWU by a few percent (for example, 205 vs. 219 at the topmost point), but this can be regarded as acceptable given the pedagogical intent. Similarly good correlation holds for other reasonable tails concentrations. The counterintuitive behavior of separative work alluded to in the Introduction originates with the logarithmic dependences in Eqs. (7) and (9); small changes in the argument of a logarithmic function, particularly if a denominator is involved, can have significant consequences. This behavior is exemplified in Fig. 4, which shows the DWU cost and product resulting from processing F = 1000 units of feed material to a given final concentration, assuming a feed concentration of fF = 0.0072 and a tails concentration of fw = 0.0025. To get to reactor-grade product of fP = 0.04 requires 710 units of work and yields only 125 units of product. Processing this product up to a bomb-grade level of fP = 0.90 requires less than half as much additional work, 309 units, but yields a paltry 5.24 units of product. If his product is kilograms of hex, it will contain only about 3.2 kg of 235U; the bare critical mass of this isotope is about 45 kg. The ratio of product to work diminishes steadily with increasing fP.
The counterintuitive behavior of separative work alluded to in the Introduction originates with the logarithmic dependences in Eqs. (7) and (9); small changes in the argument of a logarithmic function, particularly if a denominator is involved, can have significant consequences. This behavior is exemplified in Fig. 4, which shows the DWU cost and product resulting from processing F = 1000 units of feed material to a given final concentration, assuming a feed concentration of fF = 0.0072 and a tails concentration of fw = 0.0025. To get to reactor-grade product of fP = 0.04 requires 710 units of work and yields only 125 units of product. Processing this product up to a bomb-grade level of fP = 0.90 requires less than half as much additional work, 309 units, but yields a paltry 5.24 units of product. If his product is kilograms of hex, it will contain only about 3.2 kg of 235U; the bare critical mass of this isotope is about 45 kg. The ratio of product to work diminishes steadily with increasing fP.
As another way of looking at how less work is required to go from a somewhat enriched intermediate product to a highly-enriched final product than is required to get to the intermediate product to begin with, we can compute the number of particle exchanges (not draws) the demon has to execute. Suppose that we start with F = 100,000 molecules with fF = 0.0072, from which we wish to ultimately isolate product with fP = 0.90 by proceeding in two steps: First isolating product enriched to fP = 0.2, and then enriching that product to fP = 0.9. Both steps are assumed to have tails fw = 0.0025. For the first step, Eq. (3) indicates that, rounding off to the nearest whole number, P = 2380 molecules. Initially, only 0.0072(2380) = 17 of these will be of the desired light isotope. But we want to enrich this to fP = 0.2, which demands having 0.2(2380) = 476 light-isotope molecules on the P side of the chamber. Hence, the demon will have to shift 459 molecules from W to P in this step. He then executes the second step, beginning with F = 2380 and fF = 0.2. Here Eq. (3) indicates that this will require P = 524. He subdivides the new feed chamber accordingly, isolating 0.2(524) = 105 light molecules on the P side. But what is desired is 0.9(524) = 472, which will require shifting 367, or only about 80% as many actions as were required in the first step. Working through the numbers in this way can be helpful, but the ultimate metric is the work involved according as the number of drawings.
5. THE WORLD SWU SITUATION
According to the World Nuclear Association, worldwide enrichment capacity as of 2020 was about 66.7 million kg-SWU per year. [4] The European enrichment corporation Urenco makes available a menu of handy online calculators to help you estimate the optimum mix of feed and waste concentrations as a function of current kg-SWU cost.[5] According to the United States Energy Information Administration, the average per kg-SWU cost of enrichment services for civilian owner/ operator power reactors in 2021 was about $100.[6] Only a small fraction of this cost is that of the power required to run the centrifuges: one site estimates that a modern centrifuge consumes only about 50 kilowatt-hours per kg-SWU of separative work.[7] The power consumption to run a centrifuge is very modest, only about 35 Watts.[8] In short, the world has plenty of enrichment capacity.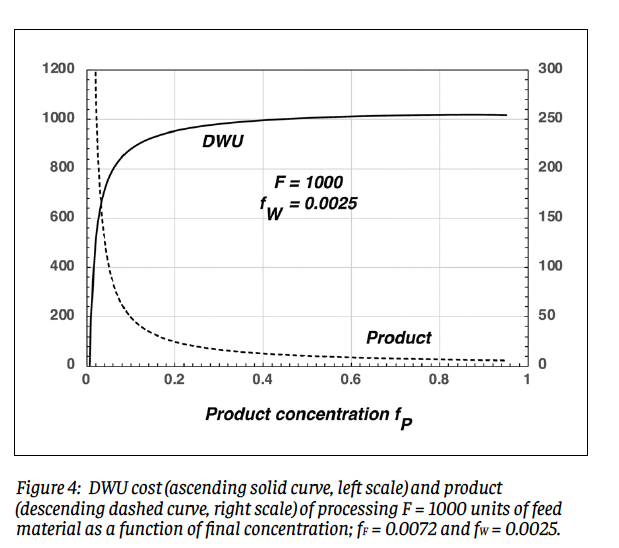
Separative work is a quantity that is conceptually straightforward, challenging to analyze rigorously, and then easy to compute once the relevant formula has been established. It is hoped that this article will help interested but non-specialist readers develop a deeper appreciation of this important issue. However current nuclear affairs resolve themselves, enrichment and its relation to potential proliferation will remain concerns for the foreseeable future.
REFERENCES
[1] K. Cohen, The Theory of Isotope Separation as Applied to the Large- Scale Production of 235U. (New York, McGraw-Hill, 1951). Available at https://www.scribd.com/doc/40224259/Karl-Cohen-The-Theory- About-Isotope-Separation
[2] S. Whitley, “Review of the gas centrifuge until 1962. Part I: Principles of separation physics,” Rev. Mod. Phys. 56(1) 41-66 (1984).
[3] J. Bernstein, SWU for You and Me. https://arxiv.org/abs/0906.2505 (2009).
[4] https://world-nuclear.org/information-library/nuclear-fuel-cycle/ conversion-enrichment-and-fabrication/uranium-enrichment.aspx
[5] https://www.urenco.com/swu-calculator
[6] https://www.eia.gov/uranium/marketing/
[7] https://www.sizes.com/units/separative work unit.htm
[8] R. L. Garwin, HEU Done It, http://fas.org/rlg/030005HDI.pdf
Top